Chicago Acid
Product Details:
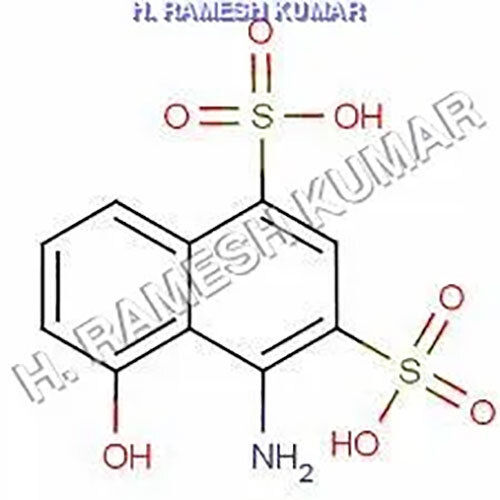
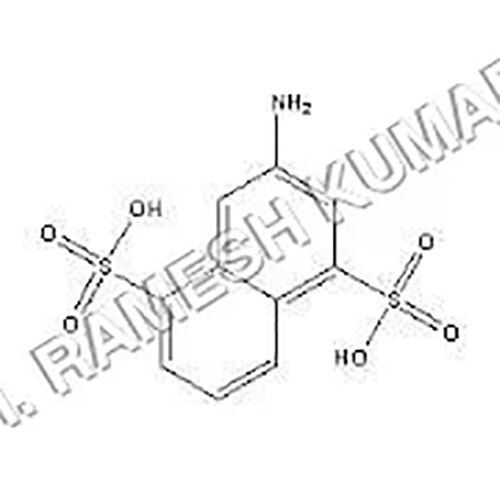
- Molecular Formula C10H8NO7S2
- CAS No 82-47-3
- Molecular Weight 318.3 Grams (g)
- Grade Industrial
- Click to View more
X
Chicago Acid Price And Quantity
- 25 Kilograms
Chicago Acid Product Specifications
- C10H8NO7S2
- 318.3 Grams (g)
- Industrial
- 82-47-3
Chicago Acid Trade Information
- Telegraphic Transfer (T/T)
- 5000 Kilograms Per Day
- 1-7 Days
- 25 kg bag
- All India
Product Description
Chicago Acid is a chemical compound that is used by analytical chemistry as a pH indicator. It is an organic compound that dissolves in water and is frequently used to assess the acidity or alkalinity of solutions with a pH between 2.0 and 4.5. ANDSA is a pH indicator used in analytical chemistry to assess the acidity of solutions. Because Chicago Acid is an azo dye, it has an azo group, a nitrogen-nitrogen double bond, connecting two aromatic rings. It has a melting point of roughly 230-240C and is a reddish-brown powder that is soluble in water.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email





 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free
